Our goal is to leverage disease models to define fundamental mechanisms that regulate lysosomal function and dysfunction. We hope these discoveries guide the development of novel therapeutic strategies aimed at restoring lysosomal health in diverse diseases.
Mapping the lysosomal degradome: a bottoms-up approach.
Studying lysosomal catabolic pathways is challenging due to their dynamic composition, acidic environment, and complex interactions with other organelles. To address this, we use organelle reconstitution and biochemical approaches to systematically map lysosomal pathways involved in macromolecule degradation. Our objective is to establish a comprehensive framework for lysosomal degradation that both extends our work on membrane proteins and goes beyond to include diverse cargos. Elucidating the molecular mechanisms that regulate the degradation of physiological substrates and pathological aggregates will enable us to pave the way for the development of new targeted therapeutics for cancer and neurodegeneration.
Cellular metabolic homeostasis and lysosomal function.
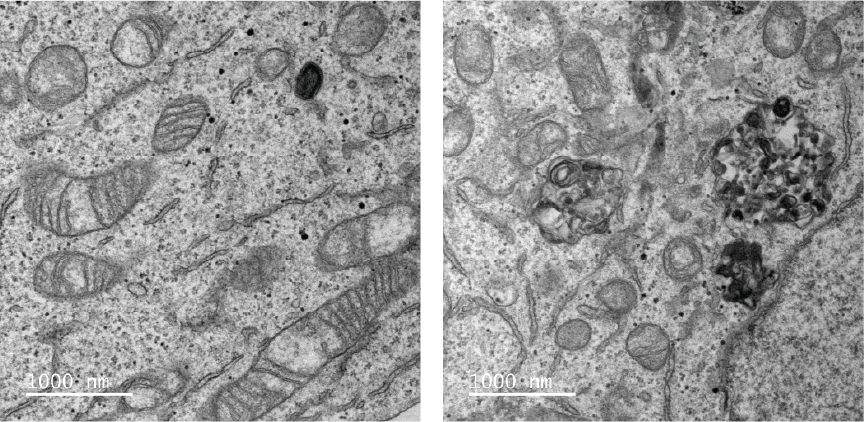
Mutations in genes encoding lysosomal hydrolases and transporters cause lysosomal storage disorders (LSDs), a spectrum of diseases that are marked by disrupted cellular metabolism, progressive neurodegeneration, developmental delay, and early death. However, the underlying pathological mechanisms remain unclear. Conversely, whether and how cellular metabolic disorders in other organelles impact lysosomal function is largely unknown. Using cellular models of LSDs and inborn errors of metabolism (IEMs), we will investigate the interplay between lysosomal function and cellular metabolic homeostasis. Our long-term goal is to uncover how metabolic inter-organelle communication supports lysosomal function and vice-versa.